Identity and Synonyms
-
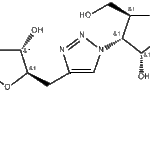
Name: 4-Dehydroxy-4-dimethylhydroxysilyl Entecavir
-
Synonym: 4-Dimethylsilyl Entecavir
-
CAS Number: 870614-82-7 clearsynth.com+2Pharmaffiliates+2
-
Chemical Formula: C₁₄H₂₁N₅O₃Si
-
Molecular Weight: Approximately 335.4 g/mol clearsynth.com+1
Structural Description
The structure of this compound is closely related to Entecavir, a known antiviral (a guanine nucleoside analogue used clinically against hepatitis B virus). In 4-Dimethylsilyl Entecavir, the 4-hydroxymethyl group of the cyclopentyl moiety in entecavir is replaced or modified to include a hydroxydimethylsilyl substituent. This makes the compound a synthetic impurity or derivative of entecavir rather than an active drug itself. clearsynth.com+2Pharmaffiliates+2
Classification and Usage Context
-
Typically categorized under drug impurities or reference standards for entecavir. Biosynth+2Acanthus Research+2
-
Provided by chemical suppliers as a quality control standard, analytical reference, or for method validation in pharmaceutical development. LGC Standards+2Biosynth+2
Functional Relevance
While entecavir is a potent anti-HBV agent, this derivative (4-Dimethylsilyl Entecavir) is not itself an approved therapeutic agent. Instead, its relevance lies in pharmaceutical analysis — particularly to:
-
Serve as an analytical standard in HPLC/LC-MS assays.
-
Assist in impurity profiling and regulatory documentation of entecavir drug products.
-
Help establish purity thresholds and detect manufacturing by-products.
Notably, due to the presence of the silicon-containing moiety, it may exhibit different retention or detection properties compared to entecavir, making it useful in distinguishing closely related compounds in chromatographic or spectrometric methods.
Summary Table
| Property | Details |
|---|---|
| Compound Name | 4-Dehydroxy-4-dimethylhydroxysilyl Entecavir (4-Dimethylsilyl Entecavir) |
| CAS Number | 870614-82-7 |
| Formula & MW | C₁₄H₂₁N₅O₃Si; ~335.4 g/mol |
| Relationship to Entecavir | Synthetic impurity / derivative |
| Primary Use | Reference standard for analytical methods; impurity profiling |
| Commercial Suppliers | Various (e.g., Clearsynth, LGC, etc.) |
| Status | Not a drug; for research use only |

If you’d like, I can also help you understand how such impurity standards are used in regulatory filings (e.g., ICH Q3A/Q3B guidelines), analytical workflows, or method development processes. Just let me know!