Definition:
PEGylation is a process of both covalent and non-covalent attachment or consolidation of polyethylene glycol (PEG) polymer chains to molecules and macrostructures, such as drugs, therapeutic proteins or vesicles. As a biocompatible and hydrophilic polymer, PEG can be covalently linked to a variety of molecules to improve their pharmacokinetics and therapeutic properties. PEGylation affects the resulting derivatives or aggregates interactions which typically slows down their polymerization, degradation and elimination in vivo. PEGylation can be achieved by the incubation of a reactive derivative of PEG with the target molecule.
PEGylation has been applied to diverse therapeutic agents, such as aptamers, peptides and even small molecules. As a result, PEGylation therapeutics are expanding the clinical pipeline of many biotech companies.
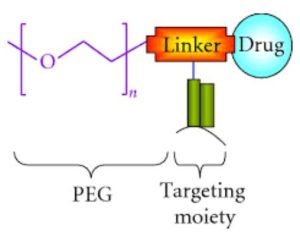
Advantages:
Extended Circulation Time: PEGylation can increase the size of the molecule, making it less prone to renal filtration and clearance. It results in a longer half-life in the bloodstream, allowing the therapeutic molecule to remain active for a longer duration.
Reduced Immunogenicity: PEGylation can reduce the immunogenicity of the molecules, with PEG acting as a shield, to prevent recognition by the immune system. It can be especially beneficial for protein-based therapeutics, which might otherwise be targeted by the immune system.
Improved Solubility: PEGylation can enhance the solubility of hydrophobic molecules, increasing their stability in solution and improving their bioavailability.
Reduced Toxicity: PEGylation can alter the pharmacokinetics of a molecule, potentially reducing its accumulation in certain tissues and minimizing side effects.
Enhanced Targeting: PEGylation can improve the selective targeting of drugs to specific tissues or cells by influencing their distribution and interaction with cellular barriers.
Improved Stability: Acting as protective barriers, PEG chains can shield the attached molecule from degradation by enzymes or other factors.
Process:
PEGylation process involves the attachment of PEG chains to a molecule of interest, such as a therapeutic protein or drug. This process can be achieved through various chemical reactions, depending on the functional groups exist in both the molecule and the PEG chains. Here’s the steps involved in the PEGylation process:
1. Selection of Molecule: Choose the molecule you would liketo PEGylate, such as a protein, peptide, small molecule drug or other biologically active compound.
2. Selection of PEG Reagents: Choose the appropriate PEG reagents based on the chemistry of the molecule and the desired PEGylation strategy.
3. Activation of PEG: The PEG molecule is typically modified or activated to owna reactive group, that can covalently bind to the target molecule. Common reactive groups include succinimidyl esters (NHS esters) for amine-reactive PEGylation and maleimide groups for thiol-reactive PEGylation.
4. Preparation of the Target Molecule: Modify the target molecule to introduce or expose reactive functional groups that will react with the activated PEG.
5. Reaction: Mix the activated PEG reagent with the target molecule under appropriate conditionsto allow the reactive groups on the PEG to react with the exposed or introduced functional groups on the target molecule, forming covalent bonds.
6. Purification: After the reaction, the PEGylated product needs to be separated from any unreacted PEG reagents, starting materials and by-products. Its is typically achieved through techniques such as size-exclusion chromatography, ultrafiltration or dialysis.
7. Characterization: Analyze the PEGylated product to confirm its identity, purity and degree of PEGylation, using thetechniques such as mass spectrometry, SDS-PAGE (Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis) and HPLC (High-Performance Liquid Chromatography) can be used for characterization.
8. Bioactivity and Stability Testing: Evaluate the PEGylated product’s bioactivity and stability to ensure that the PEGylation process has not adversely affected its therapeutic properties.
9. Formulation and Delivery: Depending on the application, the PEGylated product may need to be formulated and optimized for delivery, considering factors like solubility, stability, and release kinetics.